

Advancing The Science
At Abivax, we believe that breakthrough innovation is the key to advancing human health.
Learn how obefazimod is believed to work.
Our Scientific Journey
Novel MOA
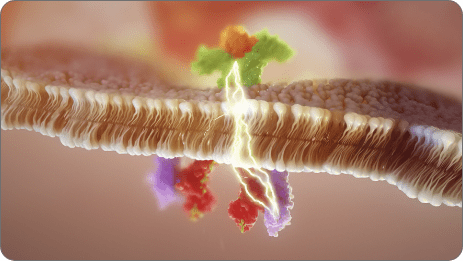
Our researchers discovered a molecule with a novel mechanism of action (MOA) that harnesses the power of microRNA biology—specifically miR-124. This molecule, obefazimod, enhances the expression of miR-124, a naturally occurring microRNA that plays a critical role in regulating the immune response.
Therapeutic Innovation
We embarked on a scientific journey to explore how this new approach could be used to treat chronic inflammatory diseases. Our discovery has provided the opportunity to bring an innovative approach to treating inflammation.
Targeting Inflammatory Diseases

Our Science
Under dysregulated inflammatory conditions in preclinical studies, enhanced expression of miR-124 resulted in stabilized levels of multiple cytokines and chemokines, bringing them back to homeostatic levels. Simultaneously restoring multiple pathways to homeostatic levels may lower the potential for compensatory immune escape mechanisms, which may result in more durable, long-term efficacy.

Our Lead Drug Candidate
Obefazimod is an investigational, orally administered, once-daily small molecule in clinical development with a novel mechanism of action. It is the first and only molecule to enhance the expression of miR-124 in immune cells, a natural regulator of the inflammatory response.
Our Research
Obefazimod demonstrated positive results in Phase 2 clinical trials, which were conducted in patients with chronic inflammatory diseases (specifically ulcerative colitis and rheumatoid arthritis).
Obefazimod is currently in Phase 3 trials (ABTECT program) in patients with moderately to severely active ulcerative colitis.
Our Pipeline
We are exploring the development of obefazimod for other inflammatory indications while simultaneously investigating follow-on compounds.
Abivax also owns a proprietary chemical library that we believe contains further untapped potential. We have launched a research program to turn that potential into a reality.