

Our scientific focus
Inflammatory Bowel Diseases (Ibd)—Chronic Diseases With No Therapeutic Cure
Inflammatory Bowel Diseases (Ibd)—Chronic Diseases With No Therapeutic Cure
IBD is a broad term that describes chronic, immune-mediated inflammatory conditions of the gastrointestinal tract with many contributing factors, including genetic, environmental, and immunologic.
The 2 most common forms of IBD are ulcerative colitis (UC) and Crohn’s disease (CD). Both diseases are generally characterized by diarrhea (blood primarily seen in UC), abdominal pain, and mucus in the stool, although there is significant heterogeneity in their clinical presentation. The two differ in where they occur and how they affect the tissue. CD can occur in every part of the GI tract and extends transmurally, while UC presents in the colon and primarily affects the colonic mucosa.
The exact cause of CD and UC remains unknown; however, a dysregulated immune system and genetic influence are possible factors.
To learn more about UC, CD, and IBD, go to:

Striving to Address an Unmet Need in IBD
IBD poses a significant health challenge:
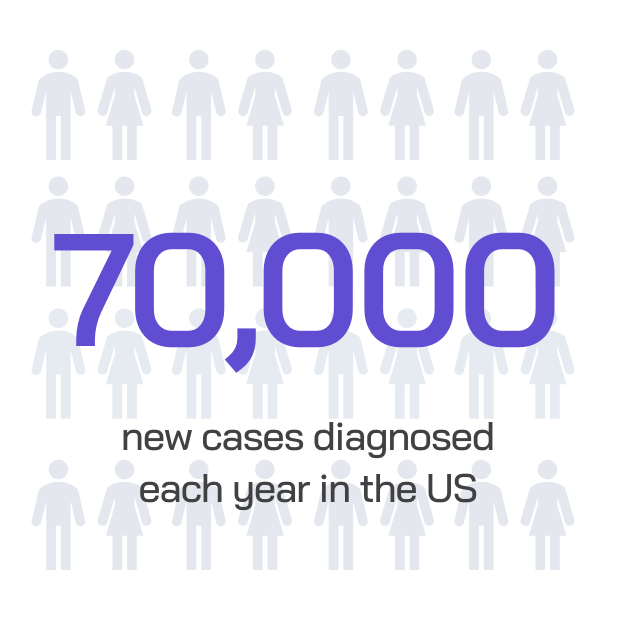
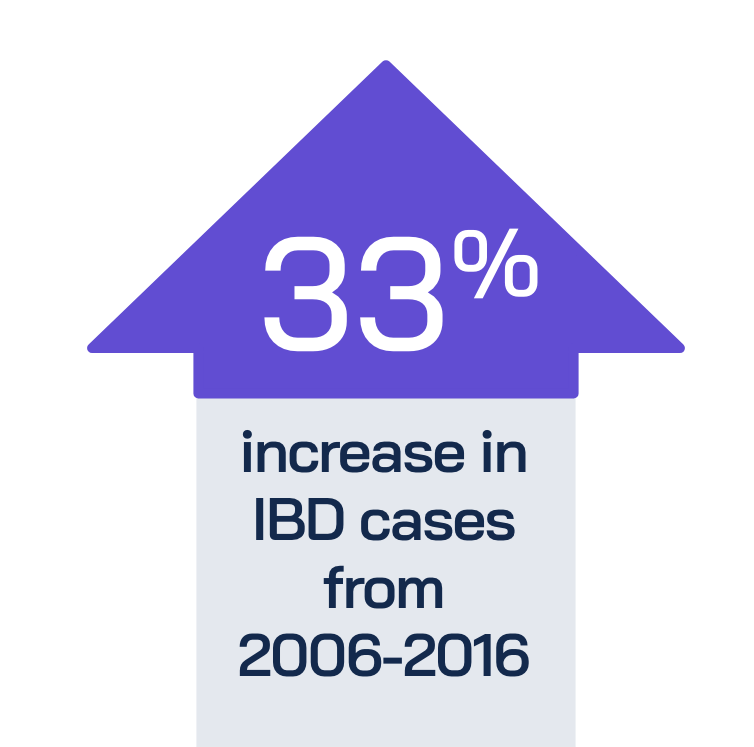

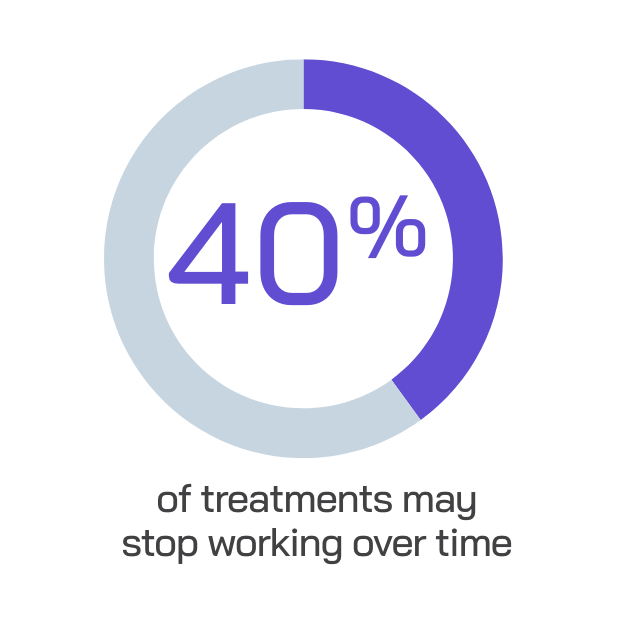
While patients with moderately to severely active IBD have many therapies available today, many traditional treatments carry potential risks and treatment burden.
We are devoted to discovering transformative treatments that harness the body’s natural regulatory mechanisms, seeking to alleviate the enduring impact that chronic inflammatory diseases can have on patients.